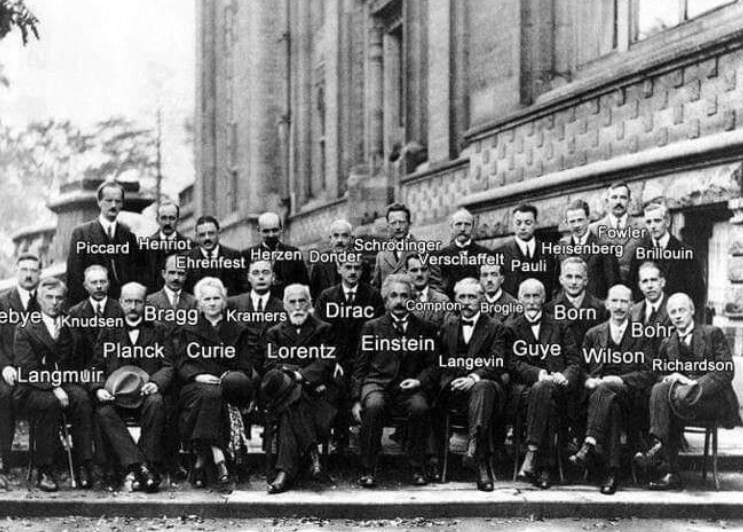
The picture was taken in Brussels in 1927 at Solvay Conference on Quantum Physics. 17 of the 29 scientists became Nobel Prize winners.
Front row (left to right): Irving Langmuir, Max Planck, Marie Curie, Hendrik Lorentz, Albert Einstein, Charles-Eugene Guye; CTR Wilson, Owen Richardson.
Middle row (left to right): Peter Debye, Martin Knudsen, William Lawrence Bragg, Hendrik Anthony Kramers, Paul Dirac, Arthur Compton, Louis de Broglie, Max Born; Niels Bohr.
Back row (left to right): Auguste Piccard, Emile Henriot, Paul Ehrenfest, Edouard Herzen, Theophile de Donder, Erwin Schrodinger, JE Verschaffelt, Wolfgang Pauli, Werner Heisenberg, Ralph Fowler; Leon Brillouin.
The Scientists in the Picture
- Albert Einstein: Einstein is undoubtedly one of the world’s most famous scientists. He earned worldwide fame for the development of the general theory of relativity and for winning a Nobel Prize in 1921 for his discovery of the law of the photoelectric effect. He is best known for his mass-energy equivalence formula, E = mc2 in Nuclear Physics, which has been dubbed “the world’s most famous equation“.
- Niels Bohr: Bohr, a Danish physicist made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922.
- Marie Curie: Marie Curie was a Polish and naturalized-French physicist and chemist who conducted pioneering research on Radioactivity. She was the first woman to win a Nobel Prize in Physics. She later won another Nobel Prize in Chemistry and she became the first person to win the Nobel prize twice. Her efforts with her husband, Pierre Curie led to the discovery of polonium and radium, and she championed the development of X-rays. Unfortunately, Marie Curie died of aplastic anaemia, believed to be caused by prolonged exposure to radiation during her many years of working with radioactive materials.
- Werner Heisenberg: Heisenberg is best known for his uncertainty principle (Heisenberg Uncertainty Principle) and theory of quantum mechanics, which he published at the age of twenty-three in 1925. He was awarded the Nobel Prize for Physics in 1932 for his subsequent research and application of this principle.
- Max Planck: Planck was a German theoretical physicist who discovered the quantum of action, now known as Planck’s constant, h, in 1900. His work laid the foundation for quantum theory, which won him the Nobel Prize for Physics in 1918.
- Louis de Broglie: De Broglie was a French physicist best known for his research on quantum theory and for predicting the wave nature of electrons. He was awarded the 1929 Nobel Prize for Physics. De Broglie’s equation on this is: Wavelength ^ = h/mv
-
Arthur Holly Compton was an American physicist who won the Nobel Prize in Physics in 1927 for his 1923 discovery of the Compton Scattering, which demonstrated the particle nature of electromagnetic radiation. He discovered that X-rays collide with electrons as if they were particles, so their frequency shifts according to the angle of deflection.
Wolfgang Paulli: Paulli was Austrian-born physicist and recipient of the 1945 Nobel Prize for Physics for his discovery in 1925 of the Pauli exclusion principle, which states that in an atom no two electrons can occupy the same quantum state simultaneously.
- Erwin Schrödinger: Schrödinger is best known for the Schrödinger equation, which describes the evolution of the wave function, a quantity that describes the wave properties of a particle.
- Hendrik Lorentz: Lorentz made significant contributions to many fields ranging from hydrodynamics to general relativity. His most important contributions were in the area of electromagnetism, the electron theory, and relativity. He discovered and gave theoretical explanation of the Zeeman Effect. He also derived the transformation equations subsequently used by Albert Einstein to describe space and time.
- Sir Owen Willans Richardson: Richardson won the Nobel Prize in Physics in 1928 for his work on thermionic emission, which led to Richardson’s Law.
- Charles Thomson Rees Wilson: Wilson reproduced cloud formation in a box. Ultimately, in 1911, supersaturated dust-free ion-free air was seen to condense along the tracks of ionizing particles. The Wilson cloud chamber detector was born.
- Charles-Eugène Guye: He was a professor of Physics at the University of Geneva. For Guye, any phenomenon could only exist at certain observation scales.
- Paul Langevin developed Langevin dynamics and the Langevin equation.
- Max Born’s probabilistic interpretation of Schrödinger’s wave function ended determinism in physics but provided a firm ground for quantum theory.
- Paul Dirac came up with the formalism on which quantum mechanics is now based. In 1928, he discovered a relativistic wave function for the electron which predicted the existence of antimatter, before it was actually observed.
- Hendrik Kramers was the first foreign scholar to seek out Niels Bohr. He became his assistant and helped develop what became known as Bohr’s Institute, where he worked on dispersion theory.
- William Lawrence Bragg was awarded the Nobel prize for physics jointly with his father Sir William Henry Bragg for their work on the analysis of the structure of crystals using X-ray diffraction.
- Martin Knudsen revived Maxwell’s kinetic theory of gases, especially at low pressure: Knudsen flow, Knudsen number etc.
-
Peter Debye pioneered the use of dipole moments for asymmetrical molecules and extended Einstein’s theory of specific heat to low temperatures by including low-energy phonons.
- Léon Nicolas Brillouin practically invented solid state physics (Brillouin zones) and helped develop the technology that became the computers we use today.
- Sir Ralph Howard Fowler supervised 15 FRS and 3 Nobel laureates. In 1923, he introduced Dirac to quantum theory.
- Jules Emile Verschaffelt, the Flemish physicist, got his doctorate under Kamerlingh Onnes in 1899.
- Théophile de Donder defined chemical affinity in terms of the change in the free enthalpy. He founded the thermodynamics of irreversible processes, which led his student Ilya Prigogine (1917-2006) to a Nobel prize.
- Edouard Herzen is one of only 7 people who participated in the two Solvay conferences of 1911 and 1927. He played a leading role in the development of physics and chemistry during the twentieth century.
- Paul Ehrenfest remarked (in 1909) that Special Relativity makes the rim of a spinning disk shrink but not its diameter. This contradiction with Euclidean geometry inspired Einstein’s General Relativity. Ehrenfest was a great teacher and a pioneer of quantum theory.
- Emile Henriot detected the natural radioactivity of potassium and rubidium. He made ultracentrifuges possible and pioneered the electron microscope.
- Auguste Piccard designed ships to explore the upper stratosphere and the deep seas (bathyscaphe, 1948).
- Irving Langmuir: Irving Langmuir was an American chemist and physicist. His most noted publication was the famous 1919 article “The Arrangement of Electrons in Atoms and Molecules”.
Info Sources: Wikipedia, Rare Historical Photos.
(Photo credit: Benjamin Couprie, Institut International de Physique de Solvay. The colored version made by u/mygrapefruit).